In the early days of chemistry, researchers used all manner of things to create a name for themselves. An example is the early isolation of the element Phosphorous which was first prepared by the distillation of decomposed horse urine from a bed of sand. Well, it works notwithstanding the probable odor and mess not to mention the difficulty of obtaining the sample. So, great effort was expended for expected great reward.
The same is true of the early days of photographic emulsion making where any and all chemicals were used in hopes that the experimenter would have that ah hah moment and discover something great. And, if they failed to produce anything significant, well they could still write a paper or book about their super dooper method of emulsion making. Some were good and some were bad. Here are some thoughts on the matter.
1. Potassium vs Sodium salts.
I saw both used at EK with no rhyme nor reason. I saw no real logic so I went to the best emulsion maker that I knew, and who was at the peak of his career. If you went any higher you might suffer nosebleed. Anyhow, I asked him which was better and why. His answer was that there was no best. Each gave a slightly different result, but there was no best and no easy way to predict what might happen if one used one salt over the other. In fact, he pointed out that most formulas originating in Europe (Kodak Harrow and Kodak Chalon) used Potassium salts and most from the US used Sodium salts. His reasoning was thus: Na salts are easier to get here in the US and are less expensive but the opposite is true in Europe. IDK if this is true and I never followed up, but after that I did see the trend of formulas from Europe did indeed use K salts more than Na salts. And, I also came to realize that there was no real way to predict what one would give compared to another, and what changes would be needed to convert from one salt to another. The bottom line here is use what you prefer, have the most of, what costs less or whatever, but stay consistent.
2. Lead, Cadmium and Mercury salts
These salts were used in many early warm tone emulsion formulas, but what goes in must come out. This means in the wash or in the process. Cadmium was the biggest offender being used anywhere from 100 mg per mole of Silver up to about 18 GRAMS / mole of silver. Cadmium and Lead have been history since about 1970, but Mercury (as a very toxic Methiodide salt) is still in use in some cases, but at very very low concentrations. It is now used as a keeping agent more than anything.
3. Ammonium salts
It had been known for years that Ammonium hydroxide dissolved Silver Nitrate to form a complex soluble material. I had been used for years in making emulsions up to the 60s when better methods were put into play. You see, this method causes fog and the effect varies with pH which varies with concentration and also during the pptn, As the Silver is precipitated, Ammonia is released and pH goes up. If it goes up too much or too fast, then fog forms. The clear advantage is that you can form uniform rounded cubic crystals which is nice. You also form a lot of fog which is bad and must be avoided by some means such as time, temperature or the use of antifoggants.
Now, some propose that Ammonium Halide salts be used, but with them, pH goes down with time during pptn. Since you are already slightly acidic, and pH goes down a bit, what you see is a decrease in the effect of Ammonia as the pptn proceeds. Well, in this case it was never very large anyhow. So, over my time at EK, I never saw a practical emulsion that used Ammonium Halides. Either Ammonium Hydrixide was used or better solvents or methods of handling the Ammonia were used. The trick, what you want, is to have the Ammonium ion present in a slightly alkaline medium and at a constant amount for the desired time. You will find this hard to do with Ammonium Halides.
4. Other positive ions
Almost every positive ion on the Periodic Chart has been used, via their halide salts or otherwise, for making Silver Halide emulsions. AFAIK, even after studying over 1000 emulsions over 15 years, only good old Na and K are still in there and still being used. All of the others have vanished due to lack of interest, cost or pollution not to mention lack of some overriding utility.
Finally, lets go back to the opening paragraphs of this post. When Phosphorous was first isolated as a pure element, the original worker considered himself a scientist. But, in view of todays chemistry he was not. In a similar fashion, emulsion chemistry was published by what we might call dabblers in the proto science of photographic science. All of the texts up to that time were done with a less than scientific method when compared with today. And so, even with the publication of the Agfa formulas in the 40s, they were published as-is with no explanation or qualification. Since then, everything has been cloaked in secrecy and all books including Glafkides and Haist have been redacted or contain outright errors in the text.
I will pass this way only one time, and during that time, I have determined that I will publish modern and correct descriptions of the science of photographic engineering as far as I am able. I had much more information before I retired, but I returned that material to EK archives when I retired. I had stacks of data that were nearly overwhelming. They would have been useful today, so all I can present is a pale comparison compared to what exists.
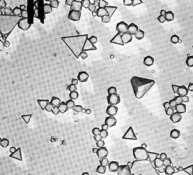
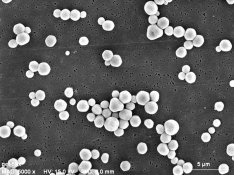
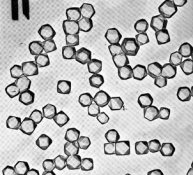
Attached is a set of electron micrographs that depict an SR (Single Run Silver Nitrate into Halide salt + gelatin) emulsion (Na or K not much difference) and an Ammonia digest using the Silver complex of Ammonia rather than NH4Br (Silver + NH4OH run into Halide salt + gelatin. The third electron micrograph is of a modern dual run emulsion in which salt and silver were run together. Note that the use of the Ammonia complex of Silver ion only approaches the quality of a dual run emulsion. Note also that an ammonium salt alone such as NH4B4 will only approach the SRAD (Ammonium complex) version very very slightly if at all!
Enjoy.
PE
The same is true of the early days of photographic emulsion making where any and all chemicals were used in hopes that the experimenter would have that ah hah moment and discover something great. And, if they failed to produce anything significant, well they could still write a paper or book about their super dooper method of emulsion making. Some were good and some were bad. Here are some thoughts on the matter.
1. Potassium vs Sodium salts.
I saw both used at EK with no rhyme nor reason. I saw no real logic so I went to the best emulsion maker that I knew, and who was at the peak of his career. If you went any higher you might suffer nosebleed. Anyhow, I asked him which was better and why. His answer was that there was no best. Each gave a slightly different result, but there was no best and no easy way to predict what might happen if one used one salt over the other. In fact, he pointed out that most formulas originating in Europe (Kodak Harrow and Kodak Chalon) used Potassium salts and most from the US used Sodium salts. His reasoning was thus: Na salts are easier to get here in the US and are less expensive but the opposite is true in Europe. IDK if this is true and I never followed up, but after that I did see the trend of formulas from Europe did indeed use K salts more than Na salts. And, I also came to realize that there was no real way to predict what one would give compared to another, and what changes would be needed to convert from one salt to another. The bottom line here is use what you prefer, have the most of, what costs less or whatever, but stay consistent.
2. Lead, Cadmium and Mercury salts
These salts were used in many early warm tone emulsion formulas, but what goes in must come out. This means in the wash or in the process. Cadmium was the biggest offender being used anywhere from 100 mg per mole of Silver up to about 18 GRAMS / mole of silver. Cadmium and Lead have been history since about 1970, but Mercury (as a very toxic Methiodide salt) is still in use in some cases, but at very very low concentrations. It is now used as a keeping agent more than anything.
3. Ammonium salts
It had been known for years that Ammonium hydroxide dissolved Silver Nitrate to form a complex soluble material. I had been used for years in making emulsions up to the 60s when better methods were put into play. You see, this method causes fog and the effect varies with pH which varies with concentration and also during the pptn, As the Silver is precipitated, Ammonia is released and pH goes up. If it goes up too much or too fast, then fog forms. The clear advantage is that you can form uniform rounded cubic crystals which is nice. You also form a lot of fog which is bad and must be avoided by some means such as time, temperature or the use of antifoggants.
Now, some propose that Ammonium Halide salts be used, but with them, pH goes down with time during pptn. Since you are already slightly acidic, and pH goes down a bit, what you see is a decrease in the effect of Ammonia as the pptn proceeds. Well, in this case it was never very large anyhow. So, over my time at EK, I never saw a practical emulsion that used Ammonium Halides. Either Ammonium Hydrixide was used or better solvents or methods of handling the Ammonia were used. The trick, what you want, is to have the Ammonium ion present in a slightly alkaline medium and at a constant amount for the desired time. You will find this hard to do with Ammonium Halides.
4. Other positive ions
Almost every positive ion on the Periodic Chart has been used, via their halide salts or otherwise, for making Silver Halide emulsions. AFAIK, even after studying over 1000 emulsions over 15 years, only good old Na and K are still in there and still being used. All of the others have vanished due to lack of interest, cost or pollution not to mention lack of some overriding utility.
Finally, lets go back to the opening paragraphs of this post. When Phosphorous was first isolated as a pure element, the original worker considered himself a scientist. But, in view of todays chemistry he was not. In a similar fashion, emulsion chemistry was published by what we might call dabblers in the proto science of photographic science. All of the texts up to that time were done with a less than scientific method when compared with today. And so, even with the publication of the Agfa formulas in the 40s, they were published as-is with no explanation or qualification. Since then, everything has been cloaked in secrecy and all books including Glafkides and Haist have been redacted or contain outright errors in the text.
I will pass this way only one time, and during that time, I have determined that I will publish modern and correct descriptions of the science of photographic engineering as far as I am able. I had much more information before I retired, but I returned that material to EK archives when I retired. I had stacks of data that were nearly overwhelming. They would have been useful today, so all I can present is a pale comparison compared to what exists.
Attached is a set of electron micrographs that depict an SR (Single Run Silver Nitrate into Halide salt + gelatin) emulsion (Na or K not much difference) and an Ammonia digest using the Silver complex of Ammonia rather than NH4Br (Silver + NH4OH run into Halide salt + gelatin. The third electron micrograph is of a modern dual run emulsion in which salt and silver were run together. Note that the use of the Ammonia complex of Silver ion only approaches the quality of a dual run emulsion. Note also that an ammonium salt alone such as NH4B4 will only approach the SRAD (Ammonium complex) version very very slightly if at all!
Enjoy.
PE


 Never let the machine run out!
Never let the machine run out!

 Good!
Good!