- Joined
- Feb 9, 2010
- Messages
- 9,455
- Format
- 4x5 Format
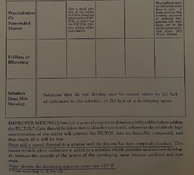
The first pinch of sulfite method dates back to The Amateur Photographers' Handbook, Aaron Sussman, as far back as I know.
It looks like advice from further back in time. The Chemistry of Photography from Mallinckrodt Chemical Works 1940 mentions it. PICTOL was their trademark for Metol