mghis
Member
- Joined
- Aug 20, 2012
- Messages
- 24
- Format
- Medium Format
Hi all.
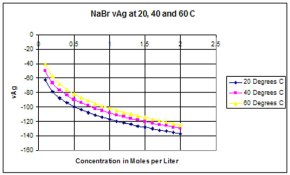
Can anyone post a table (or a graph) of solubility product of AgBr, AgI and AgCl at different temperatures? Kps at 25°C are given in most handbooks, but I have not found values at higher temperatures.
(I think I could derive them using van't Hoff equation but then again I should have to know how ΔH° varies with temperature )
Thanks!
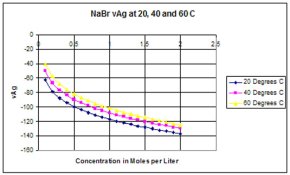
Can anyone post a table (or a graph) of solubility product of AgBr, AgI and AgCl at different temperatures? Kps at 25°C are given in most handbooks, but I have not found values at higher temperatures.
(I think I could derive them using van't Hoff equation but then again I should have to know how ΔH° varies with temperature )
Thanks!