If you say "good reduction in grain levels but a definite increase in apparent sharpness", then what is your reference developer you compare this against?
If you look at the development process, there is the initiation of development, when tiny developable silver specks start building up, and there is the rapid development phase, in which silver builds up quickly. Phase 1 is governed by the reduction potential of the developer, and by the degree to which the developer adsorbs to the silver halide grain. Phase 2 depends on these same factors, and in addition on how quickly fresh developer is brought to the grain and how quickly oxidized developer is either restored or removed from the grain.
If phase 2 is much faster than phase 1, then you quickly build up contrast, while smaller developable silver specks remain mostly undeveloped, and you see this as "speed loss".
PS: People have seen better sharpness with Metol than with Phenidone. Since you want to make an acutance developer, you could try to add small amounts of Metol to your developer. In both cases you'd have to reduce pH by quite a bit.
PPS: Since you now have brought Ascorbic Acid into your developer, make sure you have read and understood most of the stuff Ryuji Suzuki has written about this topic. Study his DS-12 developer formula and try to go from there.
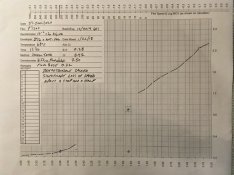
I used the wrong term actually, I'm looking more for "high sharpness and high definition", not just sharpness. Honestly fine grain and sharpness are not always at ends, just often. Specifically I believe the coarse grain I see in my sulfite-free EXG1 developer is primarily silver clumping rather than just the lack of solvent action.
Taking this theory of development, I believe what is happening is that ascorbic acid is primarily responsible for phase 1 in this developer, especially for highlights, while glycin is responsible for phase 2. The ascorbic acid oxidation products aren't really restored, but rather I believe get removed from the equation by the carbonate in solution. As far as I've researched, the only potential chemical that is capable of restoring ascorbic acid is an oxalate and that seems to only really be proven at an acidic or near neutral pH. I've messed with that combo a lot, but it gets confusing really quick without professional lab equipment and techniques for actually measuring if that property applies at alkaline pH. Regardless, it's not something in this formula. My theory is that the ascorbic acid products are sticking around too long, and preventing the glycin from developing the extreme highlights (ie, why the massively over exposed leader is less developed) and also preventing it from turning the just barely exposed grains into silver, hence the speed loss. It doesn't explain why FP4+ is so much different from Ortho 80 though. I'm beginning to suspect a camera shutter issue actually upon reviewing a few previous rolls of film. Reported exposure values by the camera's meter actually looks to be about 1/4 stop over exposed, but judging from my most recent slide film shot in the camera, it looks rather like its a 1/2 stop under exposed. Regardless, for my next batch I shot the FP4+ test strips in a different camera and am including a more diverse mix of film(FP4+, HP5+, Foma 100, T-Max 400)... Just stuck on what to tweak about the developer before processing.
I've studied Ryuji's developers and comments here extensively and actually just recently bought the final ingredient for making one of his especially interesting print developers using ascorbic + dimezone-s (could only find phenidone for a while, but Photographer's Formulary is finally back in stock). DS-12 is an interesting formula due to the mismatched amounts of ascorbic acid + metol, and the incredibly small amount of carbonate used in the working solution, and despite that still having a fairly fast development time. The TEA and salycylic acid I understand to primarily be there only for preserving the stock solution and chelating iron/copper and preventing the famous ascorbic acid "sudden death". I have both, but since I'm formulating this as an A+B solution with A containing no water (only TEA and polypropylene glycol), I think the salycylic acid isn't an important ingredient here. The TEA I'm primarily using for stock making purposes, but also for additional pH buffering and as a weak silver solvent which seems to behave quite differently from sulfite. TEA is also capable of weakly chelating some radicals (hydroquinone radicals specifically, unsure about ascorbic acid radicals) and neutralizing peroxides.
Also yes, metol definitely looks easier to work with than phenidone for this purpose if I'm going to add a third developing agent, but phenidone is super-additive with both glycin and ascorbic acid, while metol is only super additive with ascorbic acid.
At the risk of machine-gunning various sacred cattle, I strongly suspect that the two metol relatives ceased being of significant research interest to Kodak etc because Metol did the same job more efficiently (than p-aminophenol) and that glycin may have been found to be no more effective than slightly adjusting the amount of metol used (which may have depended on more accurate measuring techniques becoming available, or other analytical techniques). Kodak used pyrogallol for specific properties (tanning) in specialist developers into the 1990's, but I wonder if their avoidance of actual staining developers related to concerns over the longevity/ stability of what is effectively a dye image.
My biggest reason for not using the much cheaper and more popular metol is due to the hard requirement to maintain a much higher amount of sulfite in solution. Metol in this extremely low sulfite developer would behave similar to ascorbic acid, with the oxidation products slowing down development in heavily exposed areas. My EXG1 developer uses no sulfite at all, just TEA, carbonate, and glycin, and is capable of (at least what appears to be) full speed results, rodinal like grain, and reasonable devleopment times (almost always matching D-76 1+1)
Just to give what my thinking is with combining all of these ingredients:
* glycin -- only stable and easily usable developing agent in an extremely low sulfite formula. Also is somewhat solvent on its own. It is restored by sulfite and the oxidation products appear to simply be removed by alkali
* ascorbic acid -- Several things. Ascorbic acid is known in other formulas to potentially give a speed increase (especially with phenidone). It is not restored by sulfite and oxidation products are removed by alkali, but also neutralize the alkali. This dynamic pH juggling from this I believe can give an increase in edge effects, with contrast balanced by the glycin. The thought here is that higher density areas will have more of these very acidic oxidation products that prevent the glycin from developing near by, ie, edge effects.
* low carbonate -- too much carbonate will neutralize the ascorbic acid oxidation products too quickly to produce much edge effects
* very low sulfite -- minimal solvent effect and prevents the glycin from being restored too quickly
* bromide -- a near homeopathic amount is used. Unsure it has any effect, used it just because of some references in Film Developing Cookbook saying it can potentially increase sharpness to have a very small amount
* iodide -- Same Film Developing Cookbook recommendation. Shouldn't have much effect on cubic grain emulsions, but should increase sharpness and prevent clumping on traditional grain emulsions
* glycol -- should be effectively neutral in the developer, used to make the part A less viscous and so that the ascorbic acid dissolves more easily
* TEA -- Glycin and ascorbic acid solvent for part A. Also prevents pH from going too low, while not pushing the overall developer pH to the point that glycin and ascorbic acid really do much without the small amount of carbonate. (glycin will form a low contrast image at the TEA pH of ~9.5, but with a 1 stop loss of speed and very slow development)
Overall goal of this developer are these properties:
* "Forever" shelf life by using an A+B formulation (and a good way to use up excess glycin)
* Coarse grain and high sharpness, but without a perceived grain increase due to grain clumping (ie, I don't want the dissolved silver to be replated anywhere)
* High definition / minor edge effects, not intending to reach staining developer levels of edge effects, but something with an edge over something like Rodinal or my own EXG1
* Full speed and good results on modern high speed films, bonus points for being a decent developer for pushing
* Weakly compensating with good separation between highlights and midtones
If I can accomplish all of these goals it'd be awesome. The formula I tried is already pretty good at everything excluding speed (or maybe it is good, but not with faster modern films depending on what you consider FP4+ as), and I think edge effects could be further built up by reducing agitation.
Current modifications I'm considering are adding a very small amount of catochen to make it a true staining developer, without hopefully introducing all of the difficulties that come with a staining developer. (I can't find any info at all about catochen and glycin interaction, but with metol it is sub-additive). Remove the bromide, it may have a very minor speed effect. Increase the ascorbic acid and slightly increase carbonate to maybe increase edge effects and maybe get that ascorbic acid speed increase. Increase sulfite slightly to increase overall development rate by the glycin. Decrease TEA to increase edge effects due to less buffer and maybe increase sharpness due to less silver solvent, also decrease the expense of the developer. Increase carbonate to make development overall more rapid (12m for box speed ortho 80 is pretty slow)... Unsure exactly which direction to go, probably just going to make something up and see what happens