Hi everyone I hope you are all doing well.
I'm new here and new to cyanotypes and I am of course starting with a very complicated beginning.
I have made very thin porcelain sheets and am now trying to use them for cyanotypes. Ive got a load that I can test on but just can't seem to get it right.
I have prepared a number of them at the same time as preparing a couple of sheets of Arches WC paper. Used the same solution and left them to dry for 24 hours. The paper worked. The porcelain didn't
The paper was a yellow green before exposure, the porcelain had gone a violet colour.


Ive prepared another couple of sheets and after a couple of hours they are already going violet.(see above front and back)
I did an expose on one after only a few hours after I applied solution so it wasn't really completely dry but it has been the best result.
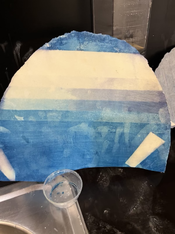
Im wondering is it possible that the porcelain is really alkaline? If so, is there anything I could do to fix that? Any other ideas?
some other


I'm new here and new to cyanotypes and I am of course starting with a very complicated beginning.
I have made very thin porcelain sheets and am now trying to use them for cyanotypes. Ive got a load that I can test on but just can't seem to get it right.
I have prepared a number of them at the same time as preparing a couple of sheets of Arches WC paper. Used the same solution and left them to dry for 24 hours. The paper worked. The porcelain didn't

The paper was a yellow green before exposure, the porcelain had gone a violet colour.


Ive prepared another couple of sheets and after a couple of hours they are already going violet.(see above front and back)
I did an expose on one after only a few hours after I applied solution so it wasn't really completely dry but it has been the best result.

Im wondering is it possible that the porcelain is really alkaline? If so, is there anything I could do to fix that? Any other ideas?
some other




 ), one should watch for changes in the properties (e.g. strength) of your ceramic after acid treatment just in case the alkali plays an important role in the properties of this material.
), one should watch for changes in the properties (e.g. strength) of your ceramic after acid treatment just in case the alkali plays an important role in the properties of this material.