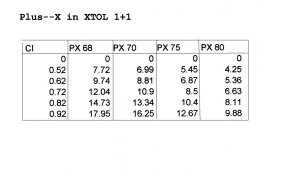
One of the most frequent questions I get about scratch mixing B&W chemicals is "how much X hydrate do I use if the formula specifies the anhydrous salt" or the reverse, "how much X do I use if the formula specifies the hydrate salt".
This is an easy question and yet hard at the same time. I'll do the easy part first.
Lets say that the formula uses 25 grams of Sodium Sulfite anh and you have the monohydrate. Well, you use 28.6 grams then.
If your formula calls for 25 grams of Sodium Carbonate anh and you have the monohydrate, then you use 29.2 grams, but if you have the decahydrate you use 43.9 grams.
How did I get that? There is a generic formula for it.
Going from ANH -> Hydrate you need a factor x ANH molecular weight.
Going from Hydrate -> ANH you need a factor X ANH molecular weight.
Whoa, where do I get molecular weight and factor? MW can be looked up on the internet and factor can be calculated. Here is how.
In the formulas below, using Kirk's suggestions...
MW = molecular weight, sometimes called formula weight
ANH = anhydrous form
HYD = hydrated form
ANH -> HYD
(MW ANH + (number of atoms of water x 18)) / MW ANH -- So, for a monohydrate the number of atoms of water is 1 and for the sulfite example above the MW is 126. The factor is then 144/126.
HYD -> ANH
MW ANH / (MW ANH + (number of atoms of water x 18)) -- so for a monohydrate to ANH conversion the factor is 126/144.
See how easy?
Now, here is the hard part.
If you consider this conversion to be important enough to ask how to convert, then you are talking about 25 grams of sulfite vs 28.6 grams of sulfite in the example I used. Easy to do on a scale, but not so easy with spoon measure. The error in spoon measures, by my direct lab experimentation is on the order of 20% either way due to crystal type and smoothing off a level spoonful, and that would mean that if you tried the above, you might get from 20 - 30 grams of sulfite no matter which salt you used.
Since sulfite controlls developer keeping and also sharpness and grain when used in film developers, I hardly think you want to mess with all of that hard work and lose it by making a 20% error, so this is the hard part.
Do you weigh out the exact number of grams or not? I doubt if there is a spoon set accurate enough to give either one of the sulfite values above, so how do you get there from here. The best you can do with a set of spoons is what they deliver when level. This may not be any of the values you need for your formula.
If the difference between anhydrous and monohydrate salts is important, then you must weigh your chemistry. If it is not, then use spoons! (again, provided that the spoons give you the values you might need, which is problematical)
Considering the number of questions I have read on both of these topics (types of salts and use of spoons and cups), I think that this is an important issue that only the individual artist can answer.
Remember, you are the judge of your own work, but I also read here of people saying this film isn't good or that film isn't good, and I also read about the complaints of lab service today due to sloppy lab techs. Do you want to be counted among them?
Do what works for you, but remember that the results are due to choices you make.
PE
This is an easy question and yet hard at the same time. I'll do the easy part first.
Lets say that the formula uses 25 grams of Sodium Sulfite anh and you have the monohydrate. Well, you use 28.6 grams then.
If your formula calls for 25 grams of Sodium Carbonate anh and you have the monohydrate, then you use 29.2 grams, but if you have the decahydrate you use 43.9 grams.
How did I get that? There is a generic formula for it.
Going from ANH -> Hydrate you need a factor x ANH molecular weight.
Going from Hydrate -> ANH you need a factor X ANH molecular weight.
Whoa, where do I get molecular weight and factor? MW can be looked up on the internet and factor can be calculated. Here is how.
In the formulas below, using Kirk's suggestions...
MW = molecular weight, sometimes called formula weight
ANH = anhydrous form
HYD = hydrated form
ANH -> HYD
(MW ANH + (number of atoms of water x 18)) / MW ANH -- So, for a monohydrate the number of atoms of water is 1 and for the sulfite example above the MW is 126. The factor is then 144/126.
HYD -> ANH
MW ANH / (MW ANH + (number of atoms of water x 18)) -- so for a monohydrate to ANH conversion the factor is 126/144.
See how easy?
Now, here is the hard part.
If you consider this conversion to be important enough to ask how to convert, then you are talking about 25 grams of sulfite vs 28.6 grams of sulfite in the example I used. Easy to do on a scale, but not so easy with spoon measure. The error in spoon measures, by my direct lab experimentation is on the order of 20% either way due to crystal type and smoothing off a level spoonful, and that would mean that if you tried the above, you might get from 20 - 30 grams of sulfite no matter which salt you used.
Since sulfite controlls developer keeping and also sharpness and grain when used in film developers, I hardly think you want to mess with all of that hard work and lose it by making a 20% error, so this is the hard part.
Do you weigh out the exact number of grams or not? I doubt if there is a spoon set accurate enough to give either one of the sulfite values above, so how do you get there from here. The best you can do with a set of spoons is what they deliver when level. This may not be any of the values you need for your formula.
If the difference between anhydrous and monohydrate salts is important, then you must weigh your chemistry. If it is not, then use spoons! (again, provided that the spoons give you the values you might need, which is problematical)
Considering the number of questions I have read on both of these topics (types of salts and use of spoons and cups), I think that this is an important issue that only the individual artist can answer.
Remember, you are the judge of your own work, but I also read here of people saying this film isn't good or that film isn't good, and I also read about the complaints of lab service today due to sloppy lab techs. Do you want to be counted among them?
Do what works for you, but remember that the results are due to choices you make.
PE
Last edited by a moderator: