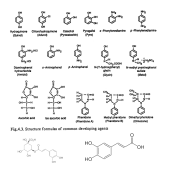
Even though I search for quite long, I haven't found an explanation for this yet.
- Ascorbic acid is NOT superadditive with chlorogenic/caffeic acid; both are in the same group as HQ (from SA point of view). So, ascorbic acid is NOT restoring the other developer agent. They are both working in parallel.
- Ascorbic acid-only developers require very high pH and are slow. SALS13, by ErikP (http://ascorbate-developers.blogspot.com/2011/03/sodium-ascorbate-sals-12.html) works at pH 13, using NaOH, and even though, development time seems to be not particulary short (let grain and contrast issues aside).
- Caffenol receipes' pH seem to be in the range 10.2-10.8, which makes sense, as they use sodium carbonate as alkali.
If all this assumptions are correct, then, ascorbic acid should not be active at the pH used in most Caffenol receipes.
But, it does have an impact. Some people wrote that it maybe reduces fog. I don't know. Some others wrote that it increases contrast (as if adding HQ to metol or phenidone). But, AFAIK, this should not be the case.
Can anyone share some thoughts about this?
- Ascorbic acid is NOT superadditive with chlorogenic/caffeic acid; both are in the same group as HQ (from SA point of view). So, ascorbic acid is NOT restoring the other developer agent. They are both working in parallel.
- Ascorbic acid-only developers require very high pH and are slow. SALS13, by ErikP (http://ascorbate-developers.blogspot.com/2011/03/sodium-ascorbate-sals-12.html) works at pH 13, using NaOH, and even though, development time seems to be not particulary short (let grain and contrast issues aside).
- Caffenol receipes' pH seem to be in the range 10.2-10.8, which makes sense, as they use sodium carbonate as alkali.
If all this assumptions are correct, then, ascorbic acid should not be active at the pH used in most Caffenol receipes.
But, it does have an impact. Some people wrote that it maybe reduces fog. I don't know. Some others wrote that it increases contrast (as if adding HQ to metol or phenidone). But, AFAIK, this should not be the case.
Can anyone share some thoughts about this?