Is there an advantage in using borax in place of sodium carbonate, assuming one achieves the same pH? - David Lyga
-
Welcome to Photrio!Registration is fast and free. Join today to unlock search, see fewer ads, and access all forum features.Click here to sign up
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
accelerator added to MQ
-
A
- Thread starter David Lyga
- Start date
Recent Classifieds
-
For Sale Darkroom Enlarger Lens SALE
- Started by J Durr
-
For Sale Mamiya 7/7ii 35mm panoramic kit unused in box
- Started by Timothy Hyde
-
For Sale Fomapan 200, 100' Bulk Roll - In Date
- Started by chuckroast
-
For Sale Kodak 2465 microfilm
- Started by MCB18
Forum statistics
- Threads
- 201,805
- Messages
- 2,830,458
- Members
- 100,964
- Latest member
- VintageLight&Shadow
- Recent bookmarks
- 0
- removed account8
- Deleted
That is what I was aiming for: is the buffering better with 20 Mule Team Borax? Actually, if you use only a little carbonate you can achieve the same pH with borax, right? - David Lyga
Depends what the developer is for, with Borax instead of Carbonate will be a poor pap[er developer.
Ian
Ian
- removed account8
- Deleted
For film development, not for paper. Sorry.
And, thank you for mentioning sodium metaborate. - David Lyga
And, thank you for mentioning sodium metaborate. - David Lyga
I have made both film and paper developers at up to pH 10.0 for my work. If buffering is an issue, I didn't see one.
In several processes, EK went from Borate to Carbonate at the same pH due to EPA regulations in some areas. Borates are toxic to citrus trees.
PE
In several processes, EK went from Borate to Carbonate at the same pH due to EPA regulations in some areas. Borates are toxic to citrus trees.
PE
- removed account8
- Deleted
I was assuming in all cases, the pH was being adjusted, which is what I tested and used.
PE
PE
No, not acid based. I just wanted to see if there was any one shot advantage using borax over carbonate, given the same developing time (ie, same pH).
David, unless you find another way to raise pH, you will see issues dissolving higher amounts of Borax, so the "it's too slow, let's add more Borax" scheme won't work. Together with Lye you could form Metaborate, which would be both highly soluble AND at the pH you really want for paper developers.
- Joined
- Nov 16, 2004
- Messages
- 3,375
I think metol quinol carbonate film developers went out of fashion in the early 1900s, the high pH causes grain.
They did exist, see p 47:
https://archive.org/stream/cu31924003618943#page/n51/mode/2up
They did exist, see p 47:
https://archive.org/stream/cu31924003618943#page/n51/mode/2up
The developer in the Kodak Tri Chem packs was a carbonate developer. It could be used for film and prints. It was still available in the 1950's. D-72 was developed as a universal developer for films and papers. It can still be usedfor film but at a higher dilution then 1+3. Ilford also had carbonate based universal developers.
Formula #6? Not that different from D-72 and Dektol.I think metol quinol carbonate film developers went out of fashion in the early 1900s, the high pH causes grain.
They did exist, see p 47:
https://archive.org/stream/cu31924003618943#page/n51/mode/2up
I do get Alan's point that MQ developers with a high concentration of carbonate are no longer used except in higher concentrations than previously was the case.
MQ Carbonate film developers were still being sold when I began darkroom work in the late 1960's. The earliest Eastman Kodak publications I have with D72 list it as a film & plate developer but it wasn't recommended for miniature formats (120 & 35mm).
I made the mistake of using D163 to process my first 120 film, this was Kodak Ltd's Uninversal developer (Dektol/D72 wasn't sold in the UK until much more recently). D163 was MQ Carbonate, the results with FP4 were grainy as expected it had been recommended by the photo dept in my local pharmacy shop.
Ian
I made the mistake of using D163 to process my first 120 film, this was Kodak Ltd's Uninversal developer (Dektol/D72 wasn't sold in the UK until much more recently). D163 was MQ Carbonate, the results with FP4 were grainy as expected it had been recommended by the photo dept in my local pharmacy shop.
Ian
- Joined
- Nov 16, 2004
- Messages
- 3,375
Until there is confirmation that it contains metol I will continue to assume that the active ingredient is pixie dust.You don't have to look back into old archives - Ilfosol 3 is based on Sodium Carbonate as well.
Ilfosol 3 is a PQ developer, it contains 1-Phenyl-4-Methyl-4-Hydroxymethyl-3-Pyrazolidone (Dimezone S) and Hydroquinone as well as Sodium Carbonate.
It's possible to use PQ Universal as a fine grain developer if used sufficiently dilute - the results are excellent, the same goes for it's MQ predecessor. PQ Universal uses Potassium Carbonate and Hydroxide.
Ian
It's possible to use PQ Universal as a fine grain developer if used sufficiently dilute - the results are excellent, the same goes for it's MQ predecessor. PQ Universal uses Potassium Carbonate and Hydroxide.
Ian
- Joined
- Nov 16, 2004
- Messages
- 3,375
Re MQ developers, by 1937 Ilford were recommending ID-11 for "Leica camera etc" and the carbonate based ID-34 was for roll films:
http://www.photomemorabilia.co.uk/Ilford/Chronology/Cezar Popescu/IlfordBookOfFormulae3rdEdition.pdf
http://www.photomemorabilia.co.uk/Ilford/Chronology/Cezar Popescu/IlfordBookOfFormulae3rdEdition.pdf
THat's about right Alan, what we forget is emulsions were changing, by 1937 Ilord had introduced the "modern" FP and HP films that eventually became FP4+ & HP5+, Kodak wee a touch later with Plus-X, Tri-X etc. Tri-X disappeared during WWII probably because it use chemicals made in Germany.
The switch to 35mm had a pround impact on film emulsions and also how they were processed, a knock on effect was paprers also had to change quite significantly to match.
Ian
The switch to 35mm had a pround impact on film emulsions and also how they were processed, a knock on effect was paprers also had to change quite significantly to match.
Ian
Tri-X disappeared during WWII probably because it use chemicals made in Germany.
There is also the possibility that all of it was being used for the war effort. I suspect that the British government had a similar demand. I remember visiting a government surplus warehouse in the early 60's. The amount of film, paper, and chemicals there was staggering. Multiply this by the fact that there was at least one such group of warehouses in each of the 50 states. Each item had its own military part number affixed. I've mentioned purchasing a case of DK-50 among other things there.
Last edited:
You're right about the Government surplus photography stores, we had a few around London all with a common partner. I used to buy my film from a couple of them in the late 60s and early 70s and visited once or twice. Huge quantities of surplus equipment, film, paper etc. At least 3 current UK suppliers have their roots in them.
Ian
Ian
- timmct
- Deleted
- Reason: Proprietary information and responses including that information.
I am interested in the use of Cl-HQ in a developer. Most every other manufacturer abandoned this in the '50s.
PE
PE
- timmct
- Deleted
- Reason: Proprietary information and responses including that information.
I am interested in the use of Cl-HQ in a developer. Most every other manufacturer abandoned this in the '50s.
PE
May & Baker were using Chloroquinone much later, well into the 1990s I;ve no idea when they stopped, they may well have been manufacturing the chemical themselves or getting it from another company in the parent group, they are now Champion.
Ian
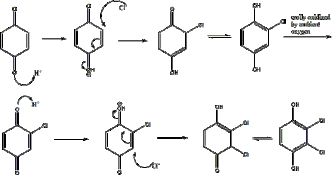
Some companies that claim to use Cl-HQ claim that they made it in-situ by adding HCl to Quinone. Well, this can only work in the absence of Oxygen. Even then it is claimed that the oxidation can be restrained by Sulfite. In the end you get either the results I've attached or you get HQMS (Hydroquinone Monosulfonate). The synthesis of Cl-HQ is actually difficult and expensive requiring the addition of Chlorine gas to Qinone in Benzene at high temperature and pressure. It is difficult to purify even then and samples that I have heard of were not pure due to decomposition.
I'm wondering if what people thought was Cl-HQ was really something else and I'm wondering what benefit they expected.
PE
I'm wondering if what people thought was Cl-HQ was really something else and I'm wondering what benefit they expected.
PE
Attachments
There's been threads on Chloroquinone, it's academic now because only low grade Chhloroquinol is manufactured.
May & Baker were probably the last company to make photograde Chloroquinol and certainly had the capabilities to achieve the purity required. They manufactured a wide variety of photo chemicals inc colour developing agents as well as many pharmaceutical and agrochemical products. M&B were mainly supplying the photographic trade.
Ian
May & Baker were probably the last company to make photograde Chloroquinol and certainly had the capabilities to achieve the purity required. They manufactured a wide variety of photo chemicals inc colour developing agents as well as many pharmaceutical and agrochemical products. M&B were mainly supplying the photographic trade.
Ian
- timmct
- Deleted
- Reason: Proprietary information and responses including that information.
-

- Photo Engineer
- Deleted
- Reason: Proprietary information and responses including that information.
- timmct
- Deleted
- Reason: Proprietary information and responses including that information.
-

- Photo Engineer
- Deleted
- Reason: Proprietary information and responses including that information.
- timmct
- Deleted
- Reason: Proprietary information and responses including that information.
| Photrio.com contains affiliate links to products. We may receive a commission for purchases made through these links. To read our full affiliate disclosure statement please click Here. |
PHOTRIO PARTNERS EQUALLY FUNDING OUR COMMUNITY:  |