Ian Leake
Subscriber
This post is for people interested in making their own ferric oxalate for platinum/palladium printing.
Most people buy their ferric oxalate from one of the specialist photographic retailers. However, I find that the best ferric oxalate is the one I make myself.
I have been working on a better way to make my ferric oxalate. It's much easier and less messy than the traditional process used by Pizzighelli and others. So far I've had very good results, with some of the best blacks I've ever made.
This process is not new to science, but doesn't seem to have made its way into the photographic community. It is based upon US Patent 1899674: “Process for the production of ferric oxalate” filed by Leo Curtin in 1933 and now expired. A more complicated version of the process was published by Dick Stevens in Making Kallitypes (1993). Stevens' process produces sulphuric acid as a by-product, so really is not suitable for small scale production.
In summary, you add oxalic acid then hydrogen peroxide to iron(ii) oxalate in water. It's easy, clean, and makes high quality ferric oxalate.
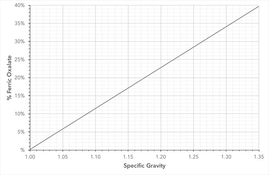
Full instructions are here on my website: https://www.ianleake.com/pages/a-modern-method-for-manufacturing-ferric-oxalate
Most people buy their ferric oxalate from one of the specialist photographic retailers. However, I find that the best ferric oxalate is the one I make myself.
I have been working on a better way to make my ferric oxalate. It's much easier and less messy than the traditional process used by Pizzighelli and others. So far I've had very good results, with some of the best blacks I've ever made.
This process is not new to science, but doesn't seem to have made its way into the photographic community. It is based upon US Patent 1899674: “Process for the production of ferric oxalate” filed by Leo Curtin in 1933 and now expired. A more complicated version of the process was published by Dick Stevens in Making Kallitypes (1993). Stevens' process produces sulphuric acid as a by-product, so really is not suitable for small scale production.
In summary, you add oxalic acid then hydrogen peroxide to iron(ii) oxalate in water. It's easy, clean, and makes high quality ferric oxalate.
Full instructions are here on my website: https://www.ianleake.com/pages/a-modern-method-for-manufacturing-ferric-oxalate