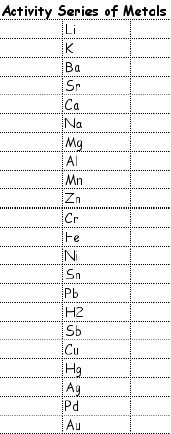
When one element replaces another element in a compound, a single replacement (also known as displacement) reaction has taken place.
The general formula for this reaction is A + BX --> AX + B.
Cu(s) + 2AgNO3(Aq) --> Cu(NO3)2(Aq) + 2Ag(s)...
The general formula for this reaction is A + BX --> AX + B.
Cu(s) + 2AgNO3(Aq) --> Cu(NO3)2(Aq) + 2Ag(s)...
You do not have permission to view the full content of this resource.
Log in or register now.